Workstream &
Work packages
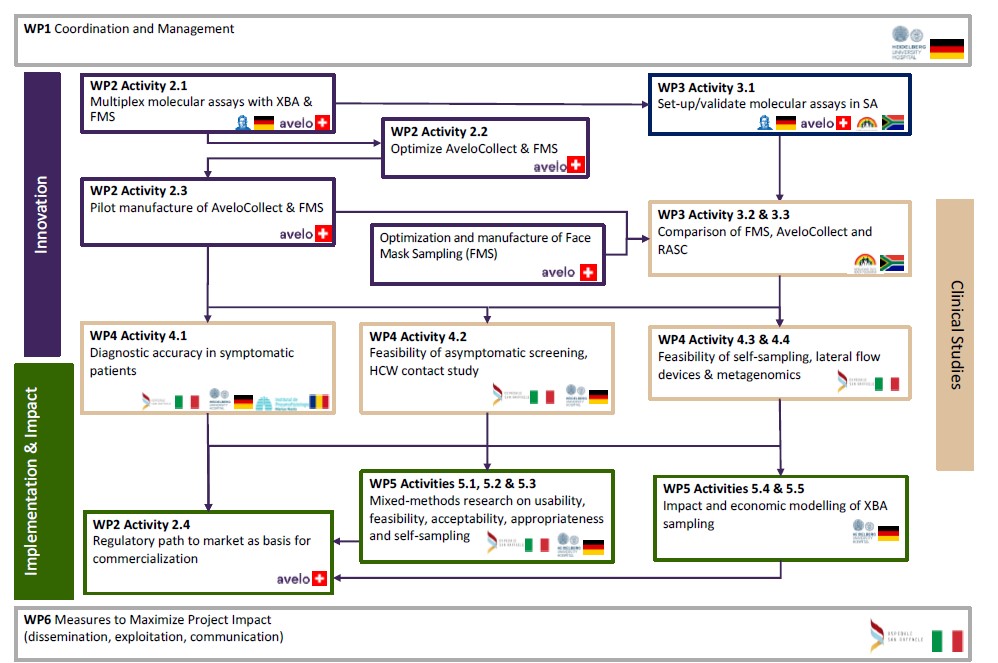
work package 1
Efficient scientific, financial, and administrative management of the project to enable the consortium to achieve proposed project goals and objectives.
The B-Path project, coordinated by UKHD, will oversee implementation, internal communication, and compliance with legal frameworks. A Project Manager will manage daily operations, supported by UKHD and in collaboration with all partners. Governance structures include a Project Executive Board, Work Package Steering Committees, and external, independent members forming the Scientific Advisory Board to ensure strategic guidance and oversight. Key deliverables include a comprehensive Data Management Plan, Publications Plan and internal Management Standard Operating Procedure. Regular meetings and robust reporting structures will track progress and deliverables to achieve project objectives and impacts.
work package 2
Optimizing the AveloCollect device in combination with molecular assays; Establishing pilot manufacturing of AveloCollect and producing AveloCollect and AveloMask for clinical studies.
Goethe University Frankfurt (GUF) will develop a multiplex molecular assay for respiratory pathogens, optimizing workflows and ensuring compatibility with AveloCollect and AveloMask. Avelo will enhance the AveloCollect device, refining materials, design, and production processes to meet clinical and regulatory standards. The device will be certified under IVDR as a Class A CE-IVD kit, and the company plans to partner with molecular diagnostics companies for broader validation and market adoption.

work package 3
Development of the Respiratory Aerosol Sampling Chamber (RASC) as a benchmark for aerosol detection of respiratory pathogens; Comparison of XBA sampling devices using the RASC.
work package 4
Evaluating the diagnostic accuracy of XBA sampling in symptomatic adults and the feasibility of XBA sampling for detecting asymptomatic infection; Exploring the feasibility of self-sampling/testing and detection of novel pathogens with viral metagenomics.
A diagnostic accuracy study will assess AveloCollect and AveloMask for detecting respiratory viruses and tuberculosis in Germany, Italy, and Romania. Over 24 months, 960 symptomatic patients will be recruited. Screening among healthcare workers and contacts will test XBA sampling for early virus detection. A self-sampling study will evaluate lateral-flow assays with XBA collection, while metagenomic analysis will validate viral detection from XBA samples, supporting its use in viral surveillance.
work package 5
Evaluating the acceptability, the usability, the population impact, and the cost-effectiveness of XBA sampling for a diagnosis and screening of respiratory infection of pandemic potential
Participant and healthcare worker (HCW) observations and interviews will inform the development of the final product, refining the configuration and sampling strategy. Mixed-methods research will assess the acceptability, usability, and appropriateness of AveloCollect and AveloMask in various healthcare settings through interviews, focus groups, and structured questionnaires. Findings will guide policy recommendations and implementation strategies. Additionally, exploratory interviews with healthcare actors in Germany will address challenges for scaling up self-sampling. An economic analysis will estimate the cost of XBA testing in both high- and low-income countries, and a compartmental model will assess TB and SARS-CoV-2 transmission with XBA screening in different epidemiological settings.
work package 6
Reaching out to society to show the impact and benefits of project activities; Dissemination and networking, making knowledge and results available to scientists, health care professionals, and others that can build upon and learn from the findings; Policy dialogue & optimal exploitation of results to facilitate their translation into new policies.
A comprehensive communication strategy will be developed, including a toolkit with branding guidelines, templates, and a public website to share project updates, achievements, and upcoming events. Short videos and social media campaigns will educate healthcare workers and the public on the XBA collection devices. Press releases, webinars, and newsletters will support outreach to the scientific community and the general public. Dissemination materials like brochures and fact sheets will be created, and an exploitation plan will outline strategies for commercializing the project’s findings. Active engagement with international organizations and participation in key conferences will promote the project’s impact. A detailed market analysis and strategy will guide the commercialization of results. A final report will summarize the findings for stakeholders to influence policy and potentially obtain WHO endorsement.





